You know a device presents a serious danger when the FDA releases not one but two warnings about the potential pelvic …


You know a device presents a serious danger when the FDA releases not one but two warnings about the potential pelvic …

The FDA issued several severe warnings about inferior vena cava filters. As a result, manufacturers recalled some IVC filters. Others …
From 2002 to 2015, the FDA received over 5,000 complaints about popular birth control device Essure. This doesn’t include the …
Deep-vein thrombosis (DVT) is a condition that affects nearly one million Americans each year. It begins when blood clots develop …
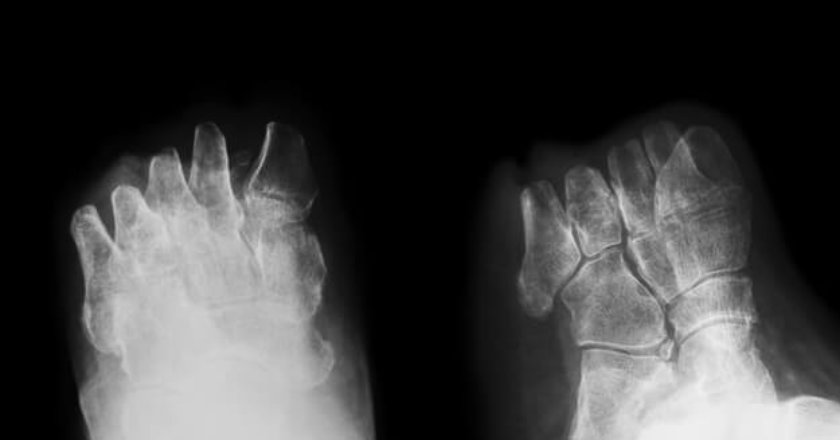
Most people who get diagnosed with type 2 diabetes don’t assume that amputation is in their future. One recent FAERS …

Many type 2 diabetes patients have trouble finding an effective glucose-controlling prescription medication that doesn’t include serious side effect risks. …
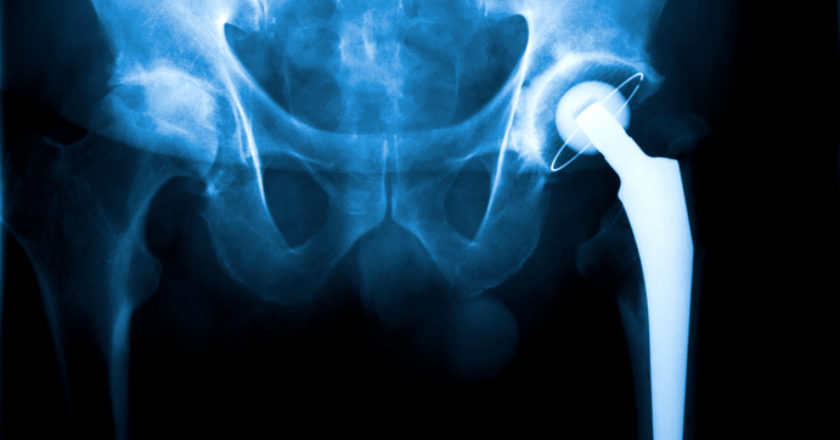
In June 2012, medical device manufacturer Stryker recalled two artificial hip implant products. This voluntary withdrawal came after the US …

You should avoid taking certain medications while you’re pregnant—and the list is very long. In the past, the FDA has …
Earlier in 2014, the U.S. Food and Drug Administration sent a warning letter to American Medical Systems (AMS) following a growing number of lawsuits against the company due to faulty transvaginal mesh devices (TVM), and a statement from parent company Endo International PLC announced that AMS will move forward with the 522 postmarket surveillance study for the Elevate product.
Endo International PLC., the parent company of American Medical Systems (AMS), was issued a letter of warning from the U.S. Food and Drug Administration (FDA) following an inspection of the facility where it found three federal regulation violations against how some urological devices and transvaginal mesh are manufactured.

Xarelto is a blood thinner, or anticoagulant, that’s prescribed to help prevent heart attacks in people who are at risk, but it also carries the possible side effect of internal bleeding that often leads to hospitalization.

You know about food poisoning and lead poisoning, but what about metal poisoning? This rare condition is known as metallosis, …

During a flare-up, people with severe heartburn will usually try anything to relieve the pain. Dietary changes and weight control …

Invokana is an oral SGLT2 inhibitor medication used to treat type 2 diabetes. It was first released in 2013 and …
If you were recently diagnosed with cancer, comparing chemotherapy drugs is probably the last thing on your mind. But with …


Acid flowing back into the esophagus causes heartburn. It can be extremely painful. Heartburn’s a symptom of gastroesophageal reflux disease (GERD). Of …