Xarelto Settlement & Financial Compensation Information
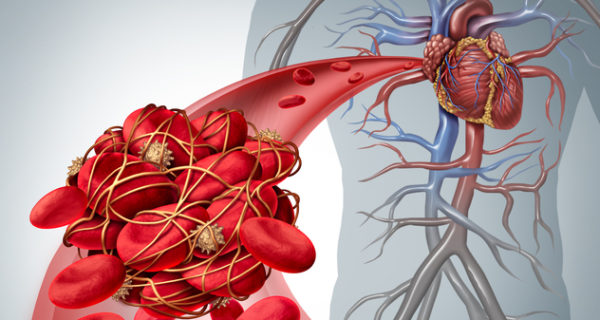
 Xarelto® (generic name: rivaroxaban) is a prescription blood thinner that helps reduce stroke and blood clot risks. Unfortunately, many Xarelto patients have already been hospitalized due to serious internal bleeding. At 2018’s close, at least 25,000 Xarelto lawsuits were pending against Johnson & Johnson and Bayer. To avoid costly ongoing litigation, the two companies agreed to pay a multimillion-dollar Xarelto settlement in spring 2019.
Xarelto® (generic name: rivaroxaban) is a prescription blood thinner that helps reduce stroke and blood clot risks. Unfortunately, many Xarelto patients have already been hospitalized due to serious internal bleeding. At 2018’s close, at least 25,000 Xarelto lawsuits were pending against Johnson & Johnson and Bayer. To avoid costly ongoing litigation, the two companies agreed to pay a multimillion-dollar Xarelto settlement in spring 2019.
Pradaxa, Xarelto Lawsuits Share Eerily Similar Injuries & Allegations
In June 2014, a Florida widow filed a lawsuit claiming her husband died of a subdural hemorrhage while taking Xarelto. Her husband started taking Xarelto in January 2012 to treat atrial fibrillation, then suffered the subdural hemorrhage in June 2013. Despite medical treatment, he died five days later. Her lawsuit also states that “Defendants concealed their knowledge that Xarelto® can cause life threatening, irreversible bleeds from the Decedent, other consumers, the general public, and the medical community.” Stuntebeck and other plaintiffs say Xarelto’s manufacturer didn’t properly inform doctors about uncontrolled bleeding risks. They also say Xarelto’s marketing is inaccurate as well as misleading. As anyone can see, plaintiffs filing claims against Pradaxa and Xarelto share eerily similar injuries as well as lawsuit allegations.
In October 2015, the U.S. Food and Drug Administration granted accelerated approval for Boehringer’s reversal agent, Praxbind® (idarucizumab). During emergency surgery or an uncontrolled bleeding episode, Pradaxa patients now have an antidote. While both medications are Factor Xa inhibitors, Praxbind doesn’t work on Xarelto patients. However, in May 2018, the FDA finally approved AndexXa, the first reversal agent that helps stop excessive bleeding in Xarelto and Eliquis patients.
Spring 2019: Bayer, Johnson & Johnson Agree to Pay $775 Million Xarelto Settlement
On March 25, 2019, J&J and Bayer (which jointly sell the blood thinner) agreed to pay a $775 million Xarelto settlement. Without admitting liability, both companies agreed to split Xarelto settlement payments aimed at resolving 25,000 pending lawsuits. Xarelto, Bayer’s best-selling pharmaceutical drug, generated over $4 billion in sales revenue for 2018 alone. This landmark Xarelto settlement helps deliver the justice and compensation injured plaintiffs deserve. It also sends a message to Big Pharma: Put patient safety above your profit margins!
If you were hospitalized for internal bleeding while taking Xarelto, you may qualify for a cash settlement. To see if you may qualify for financial compensation, complete your free case review today. Once you’ve submitted your information, an experienced lawyer will call to discuss your case.
Check eligibility for compensation.
If you or a loved one suffered life-threatening Xarelto side effects (including fatal bleeds), you may qualify for compensation from the manufacturer. Request your free case evaluation now to see if you may qualify.