Xarelto Side Effects & Health Complications
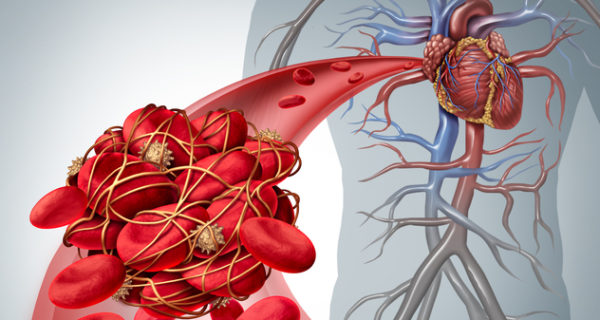
 Xarelto® (rivaroxaban) helps prevent stroke and blood clots in pulmonary embolism and deep vein thrombosis patients. Unlike warfarin, Xarelto blood thinner patients don’t need monthly monitoring. However, many Xarelto patients risk uncontrolled internal bleeding episodes. Unfortunately, some Xarelto patients require hospitalization and even bleed out. Now, injured patients are suing the drug’s manufacturer over these serious Xarelto side effects. In addition, plaintiffs say the drug’s manufacturers didn’t properly warn doctors about how to handle uncontrolled bleeding episodes.
Xarelto® (rivaroxaban) helps prevent stroke and blood clots in pulmonary embolism and deep vein thrombosis patients. Unlike warfarin, Xarelto blood thinner patients don’t need monthly monitoring. However, many Xarelto patients risk uncontrolled internal bleeding episodes. Unfortunately, some Xarelto patients require hospitalization and even bleed out. Now, injured patients are suing the drug’s manufacturer over these serious Xarelto side effects. In addition, plaintiffs say the drug’s manufacturers didn’t properly warn doctors about how to handle uncontrolled bleeding episodes.
How Xarelto Side Effects Compare to Other New Wave Blood Thinners
The Food and Drug Administration approved rivaroxaban (Xarelto) in 2011 to prevent strokes in atrial fibrillation patients. Xarelto’s closest rival is another newer anticoagulant, Pradaxa® (dabigatran), which carried similar uncontrolled bleeding risks. A 2012 report from the Institute for Safe Medication Practices found Xarelto’s adverse events surpassed Pradaxa’s. The report attributes increasing adverse events to doctors writing more Xarelto prescriptions.
“Total dispensed outpatient prescriptions for rivaroxaban have rapidly increased to nearly one million prescriptions per quarter, while dabigatran utilization has steadily declined since a peak in early 2012,” said IMSP. In fact, doctors prescribed Xarelto blood thinner almost twice as often as Pradaxa in 2013. Both anticoagulants apparently require less blood monitoring than Coumadin, making them warfarin’s likely successors. And while Vitamin K easily reverses Coumadin’s blood-thinning effects, no Xarelto antidote existed until May 2018. Prior to that, any internal bleeding episode or minor injury could kill Xarelto patients. As a result, many families filed wrongful death lawsuits against the drug’s manufacturer, Johnson & Johnson, and its subsidiary, Janssen Pharmaceuticals.
Xarelto Side Effects During Pregnancy
A Canadian study published in the American Journal of Obstetrics and Gynecology observed rivaroxaban (Xarelto) use among pregnant women. According to Everyday Health, pregnancy makes women six times more likely to develop deep vein thrombosis. Often, physicians prescribe blood-thinning medication to pregnant women. However, researchers now say Xarelto may not be the best choice.
The study reported that rivaroxaban rapidly crossed the placental barrier in both maternal-to-fetal and fetal-to-maternal directions over three hours. In other words, taking Xarelto while pregnant may potentially harm your baby. But the study also notes that since rivaroxaban is highly bound to plasma proteins, it could significantly reduce how much Xarelto reaches the fetus. As a result, researchers recommend additional studies on Xarelto use in pregnant women to better understand its risks.
The drug’s warning label says Janssen didn’t conduct any Xarelto safety studies on pregnant women. Still, it does say patients can use the drug if potential benefits outweigh any risks. Pregnant women worried about Xarelto side effects (like uncontrolled bleeding) might prefer a different medication.
Lawsuit Cites Insufficient Warnings About Xarelto Side Effects
In July 2014, 71-year-old Jeanne Jeffcoat sued Janssen for failing to adequately warn consumers about dangerous Xarelto side effects. Jeffcoat took Xarelto for just four months before checking into the hospital with uncontrollable bleeding in July 2012. Her lawyers say Jeffcoat’s bleeding episode caused permanent injuries, pain, suffering. Due to complications from her Xarelto side effects, Jeffcoat now needs ongoing medical care.
According to ISMP’s QuarterWatch, Xarelto patients in the U.S. report more fatal, disabling or serious injuries to the FDA than any other drug. Unfortunately, many rivaroxaban patients die during severe or internal bleeding episodes.
Bayer, J&J Agree to Pay $775 Million to Settle 25,000 Claims Over Life-Threatening Xarelto Side Effects
If you or a loved one took Xarelto and required hospitalization for internal bleeding, you may qualify for compensation. Victims’ family members may also wish to file a wrongful death lawsuit due to fatal Xarelto side effects. On March 25, 2019, Bayer and J&J agreed to pay $775 million to settle 25,000 lawsuits over Xarelto side effects. While neither company admitted liability, they will split payments down the middle in order to avoid ongoing costly litigation. This is a huge victory that delivered both justice and financial compensation to plaintiffs that suffered Xarelto side effects.
To check your eligibility for a cash settlement, click the button below now. Time to claim any money from this settlement agreement is limited, so don’t wait! Act now to see if your case may qualify for some Xarelto settlement money before it’s too late.
Check eligibility for compensation.
If you or a loved one experienced blood clots, uncontrolled bleeding or death while taking Xarelto, you may qualify for financial compensation from the manufacturer. Request your free case evaluation now to see if you may qualify.