Filing a Stryker Hip Replacement Claim
 If you or a loved one suffered health complications due to a faulty LFIT Anatomic CoCr V40™ Femoral Head hip implant, you may have a Stryker hip replacement claim. Plaintiffs filing a Stryker hip replacement claim allege the device’s manufacturer:
If you or a loved one suffered health complications due to a faulty LFIT Anatomic CoCr V40™ Femoral Head hip implant, you may have a Stryker hip replacement claim. Plaintiffs filing a Stryker hip replacement claim allege the device’s manufacturer:
- Knowingly marketed and sold a medical device prone to premature failure after implantation
- Is guilty of negligence for failing to adequately safety-test the device before market approval
- Committed breach of warranty
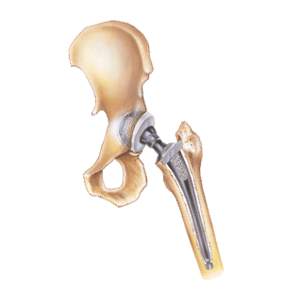
Stryker requested approval for the Stryker LFIT Anatomic CoCr V40™ Femoral Head via the FDA’s 510(k) form in 2001. Using a 510(k) form expedites approval for “substantially equivalent” medical devices. In other words, Stryker didn’t conduct any clinical human safety trials before bringing the LFIT V40 to market. Interestingly, the Stryker LFIT V40 Femoral Head contains a proprietary titanium alloy. This is the same material found in recalled ABG II and Rejuvenate hip implants that leaked metal into patients’ bloodstreams. These modular, interchangeable hip replacement components are prone to premature fracture due to corrosion. It can also trigger spontaneous dislocation, dissociation, metallosis, shortening of limbs and chronic pain, among other life-threatening side effects. If serious complications occur, the patient eventually requires revision surgery to repair any damage.
A 2012 study published in Orthopedics found that 92.5% of metal-on-metal hip implant patients require revision surgery within three years. However, Stryker’s website lists hip implant components with a 13-15 year lifespan. Research shows the need for revision surgery within three years, but recent claimants say they didn’t know about this risk. As a result, many choose to file a Stryker hip replacement claim that includes failure to warn and product liability allegations. The high hip implant failure rate for Stryker LFIT V40 femoral heads triggered a voluntary recall in late 2016.
Stryker Settled Lawsuits Over Recalled Rejuvenate and ABG II Stems
In 2014, Stryker agreed to pay $1.43 billion to plaintiffs whose Rejuvenate and ABG II modular-neck stems failed prematurely. Those patients needed revision surgery for implants recalled in July 2012 due to fretting and corrosion at the modular-neck junction. Thousands of litigants cited swelling, pain, local tissue necrosis and infection as well as other Stryker hip replacement side effects. These complications arose from the devices’ chromium and cobalt neck components rubbing against their titanium-coated stems, which released metallic debris into the patient’s bloodstream. This can lead to serious health complications such as metal poisoning, dissolution of bone tissue, taper lock failure and chronic pain. Each plaintiff won $300,000 in damages, which may offer hope for those with a currently pending Stryker hip replacement claim.
Find the Right Attorney Before Filing a Stryker Hip Replacement Claim
If either you or a loved one needed revision surgery, you may wish to file a Stryker hip replacement claim. An experienced mass tort lawyer can review your medical history to see if you may have a claim. Thorough documentation helps determine whether your injury qualifies for a cash settlement from the manufacturer. To see if you may qualify for financial compensation due to your injuries, click the button below now to start your free claim evaluation. Once you’ve submitted your information, an experienced local lawyer will call to explain your compensation options.
Check your eligibility for compensation.
If you or a loved one received a Stryker LFIT V40 hip replacement implant and later experienced life-threatening complications, you may be entitled to compensation from the manufacturer. Request your free case evaluation now to see if you may qualify.