Stryker Hip Replacement Overview - Modular Implants
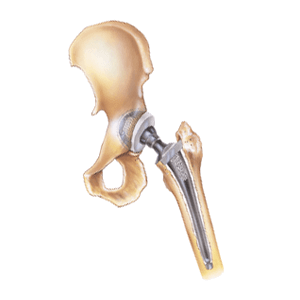 An artificial hip can relieve pain and may restore mobility in patients with arthritis, hip disease or other joint injuries. Manufacturers now create hip implants in a variety of sizes, materials, and dimensions designed to fit each patient’s anatomy. While many total hip replacement systems are monoblock designs (i.e., one connected unit), Stryker makes modular implants. Surgeons use their components in partial as well as total hip replacement surgery. Stryker manufactures each implant using a proprietary titanium TMZF alloy containing iron, zirconium and molybdenum. The company devised this proprietary blend to resist corrosion and fretting, which causes metal-on-metal grinding as well as metallosis. Flexible designs like these help surgeons custom-fit implants to closely resemble a patient’s anatomy. Learn more about the controversy surrounding these modular implants in our Stryker hip replacement overview.
An artificial hip can relieve pain and may restore mobility in patients with arthritis, hip disease or other joint injuries. Manufacturers now create hip implants in a variety of sizes, materials, and dimensions designed to fit each patient’s anatomy. While many total hip replacement systems are monoblock designs (i.e., one connected unit), Stryker makes modular implants. Surgeons use their components in partial as well as total hip replacement surgery. Stryker manufactures each implant using a proprietary titanium TMZF alloy containing iron, zirconium and molybdenum. The company devised this proprietary blend to resist corrosion and fretting, which causes metal-on-metal grinding as well as metallosis. Flexible designs like these help surgeons custom-fit implants to closely resemble a patient’s anatomy. Learn more about the controversy surrounding these modular implants in our Stryker hip replacement overview.
Stryker Hip Replacement Overview: Device Recall History
Unfortunately, high failure rates mean Stryker hip replacement patients may need revision surgery. As a result, in August 2016, Stryker voluntarily recalled all LFIT V40 implants made before 2011. The Stryker LFIT CoCr V40™ femoral head is a prosthetic ball attached to a stem embedded in the patient’s femur. (The stem may also be made of TMZF alloy with a PureFix HA coating.) Back in 2012, Stryker recalled its Rejuvenate and ABG II modular-neck hip stems. Rejuvenate and ABG II patients had premature corrosion and fretting issues, like swelling, inflammation, and taper lock failure. Then in 2014, Stryker paid $1.43 billion to settle thousands of Rejuvenate and ABG II lawsuits.
Stryker Hip Replacement Overview: LFIT V40 Patients May Need Revision Surgery
Taper lock failure (e.g., the connection between the femoral head and neck breaks) requires immediate revision surgery. Since Stryker hip replacement systems were supposed to last over a decade and failed a few years after implantation, patients with LFIT V40 femoral heads may need revision surgery. Notably, a 2012 Orthopedics study found 9 out of 10 metal-on-metal hip implants require revision surgery within three years.
Stryker Hip Replacement Overview: Injured Plaintiffs File LFIT V40 Lawsuits
Just months before Stryker settled the Rejuvenate and ABG II claims in 2014, injured patients filed at least five LFIT V40 lawsuits. New Jersey plaintiffs injured after receiving LFIT V40 femoral heads allege the company failed to adequately warn patients about hip implant corrosion and metal toxicity risks. In some cases, spontaneous dislocation and dissociation signaled premature hip replacement failure.
In July 2016, Annah Marie Gidora filed another LFIT V40 Stryker hip replacement lawsuit one month before the voluntary recall. Gidora’s claim accuses Stryker of negligence and violating New York’s consumer protection laws, among other allegations. Then in November, a man requiring LFIT V40 femoral head revision surgery filed yet another Stryker hip lawsuit. The Massachusetts plaintiff suffered soft tissue necrosis, implant corrosion and metallosis. He alleges all V40 femoral heads are defective and that Stryker intentionally downplayed any failure risks.
Many Stryker hip replacement lawsuits are still pending, and the similarities among ABG II and Rejuvenate claims are striking. All three modular hip implant systems had unexpectedly high premature failure rates. However, whether Stryker will pay to settle LFIT V40 claims before trial or wait for a court ruling remains unclear.
Check your eligibility for compensation.
If you or a loved one received a Stryker LFIT V40 hip replacement implant and then experienced life-threatening complications, you may qualify for compensation from the manufacturer. Request your free case evaluation now to see if you may qualify.