Stryker Hip Replacement Lawsuit Overview
 Several lawsuits are now pending against Stryker Corporation, maker of the Stryker LFIT Anatomic CoCr V40™ Femoral Head hip implant. Due to high adverse reaction reports, Stryker recalled the cobalt and chromium alloy hip implant in August 2016. Plaintiffs filing Stryker hip replacement lawsuit claims allege the implant’s defective design caused serious health complications. Patients with V40 Femoral Head implants manufactured before 2011 may experience severe pain, limb shortening, revision surgery necessitated by corrosion or breakage, infection, and tissue necrosis. If you received a Stryker hip implant between 2011 and 2016 and needed revision surgery afterwards, you may be eligible for compensation.
Several lawsuits are now pending against Stryker Corporation, maker of the Stryker LFIT Anatomic CoCr V40™ Femoral Head hip implant. Due to high adverse reaction reports, Stryker recalled the cobalt and chromium alloy hip implant in August 2016. Plaintiffs filing Stryker hip replacement lawsuit claims allege the implant’s defective design caused serious health complications. Patients with V40 Femoral Head implants manufactured before 2011 may experience severe pain, limb shortening, revision surgery necessitated by corrosion or breakage, infection, and tissue necrosis. If you received a Stryker hip implant between 2011 and 2016 and needed revision surgery afterwards, you may be eligible for compensation.
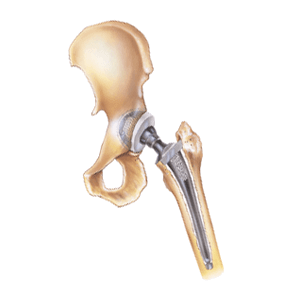
What Is a Stryker LFIT V40 Femoral Head Hip Implant?
The Stryker Low Friction Ion Treatment (LFIT) V40 Femoral Head is an interchangeable, ball-shaped hip implant. Initially approved for use in 2006, it is 20% stronger than competing titanium alloy devices. It was also designed to enhance hip stability, minimizing dislocation, and maximizing range of motion for patients undergoing hip arthroplasty. A surgeon screws the LFIT V40 Femoral Head onto an artificial hip stem’s femoral neck. The taper lock is where a hip implant’s femoral head connects to a stem inserted into the patient’s femur. This connection helps the implant move and rotate within the patient’s hip socket as naturally as possible. The Stryker LFIT V40 is designed to avoid the metal-on-metal grinding found in traditional hip replacement implants. However, it still shows higher-than-expected taper lock failure, metal corrosion and toxicity rates in patients.
Stryker Hip Replacement Lawsuit History: Failures Spur Device Recalls
Stryker settled two previous lawsuits for recalled modular-neck hip stems (Rejuvenate and ABG II). These hip implant systems with interchangeable components were approved by the FDA in 2008 (Rejuvenate) and 2009 (ABG II). Both previously recalled hip stems produced metal-on-metal grinding within patients’ bodies, which can cause a condition called metallosis. Other reported complications included corrosion, pain, swelling and inflammation in the surrounding tissue, premature hip implant failure and metal poisoning. The unexpectedly high number of adverse reactions and premature hip replacement failures triggered a device recall in 2012. However, thousands of patients with corrosion and fretting around the modular neck junction still needed revision surgery. As a result, Stryker agreed to pay $1.43 billion in 2014 to patients needing revision surgery for prematurely failing hip implants. Each plaintiff won approximately $300,000 in damages.
Current Stryker Hip Replacement Lawsuit Information
A man with metal blood poisoning and implant corrosion filed a product liability lawsuit against Stryker in November 2016. The Massachusetts man required revision surgery because his Accolade TMZF Hip Stem and LFIT V40 Femoral Head prematurely failed. After artificial hip replacement surgery in 2009, he felt constant pain and eventually developed significant soft tissue necrosis in 2015. The defective device’s stem/head juncture fractured prematurely due to years of corrosion. At least five patients with premature LFIT V40 Femoral Head failures filed similar lawsuits since 2014.
More patients with premature implant failure may file a Stryker hip replacement lawsuit going forward. If you received a Stryker hip replacement between 2011 and 2016, you may have a case. Defective implants can cause serious health complications, such as: dislocation, loss of mobility, chronic pain, metal poisoning as well as revision surgery. Stryker’s eagerness to settle prior hip replacement lawsuits before trial may offer hope for LFIT V40 implant claims.
To check your own eligibility for a Stryker hip replacement lawsuit cash settlement, click the button below now. Once you’ve submitted your information, an experienced attorney will call to discuss how to get the justice and compensation you deserve.
Check your eligibility for compensation.
If you or a loved one experienced life-threatening complications from a Stryker hip replacement, you may qualify for compensation from the manufacturer. Request your free case evaluation now to see if you may qualify.